Abstract
Background: Chimeric antigen receptor T (CAR T)-cell therapy has led to unprecedented responses in patients with relapsed/refractory non-Hodgkin's lymphoma and heavily treated multiple myeloma. Despite impressive and relatively durable response rates, CAR T-cell therapy does not work for all patients and most patients tend to relapse. Recent correlative data from patients with decade-long remissions after CAR T-cell therapy showed that the initial response phase is driven by CD8+ CAR T-cells with a long-term remission phase dominated by CD4+ CAR T-cell phenotypes [Melenhorst JJ et al. Nature, 602(7897), 503-509]. Preclinical data has shown that G-CSF directly influences the effector function of cytotoxic CD8+ T cells and may lead to reduced reactivity [Franzke A et al. (2003) Blood, 102(2), 734-739 & Bunse CE et al. (2016). Clinical & Experimental Immunology, 185(1), 107-118]. Studies outlining the prognostic impact of G-CSF among patients treated with CAR T-cell therapy remain limited. There are no consensus guidelines for management of prolonged cytopenia using growth factor support, with wide variations in guiding practice amongst physicians or institutions.
In this study, we recorded the utilization of G-CSF in a cohort of 80 consecutive patients treated with CAR T-cell therapy at our institution. We analyzed its potential impact on treatment response and survival after CAR T-cell therapy.
Methods: This is a retrospective analysis of consecutive adult patients with an underlying B cell non-Hodgkin lymphoma (n=60) or multiple myeloma (n=20) treated with CAR T-cell therapy from 2016-2022. Baseline characteristics, laboratory measurements and clinical events following CAR T-cell therapy were collected by review of patients' electronic medical record. Medication records were reviewed to determine exposure to G-CSF, starting on day +1 through day +30 following CAR T-cell infusion. We used a chi square test or Fisher's exact test to compare proportions between groups. We calculated unadjusted and adjusted hazard ratios (HR) and 95% confidence intervals (CI) for overall survival (OS) and progression-free survival (PFS) and compared survival between the groups using the Mantel Cox log rank test.
Results: Of 80 patients treated with CAR T-cell therapy, 76.2% (61/80) developed neutropenia with nadir ANC <500/uL before day +30. The median time to onset of neutropenia was +1 day, and 26.2% (21/80) of patients were neutropenic at the time of CAR T-cell infusion. In this cohort, 68.7% (55/80) of patients received G-CSF for management of severe neutropenia between days +1 and +30. Median time to initiation of G-CSF was 4 days (range 1-23 days) after treatment. Use of G-CSF did not decrease median length of stay compared to patients who did not receive G-CSF (20.5 vs 19 days).
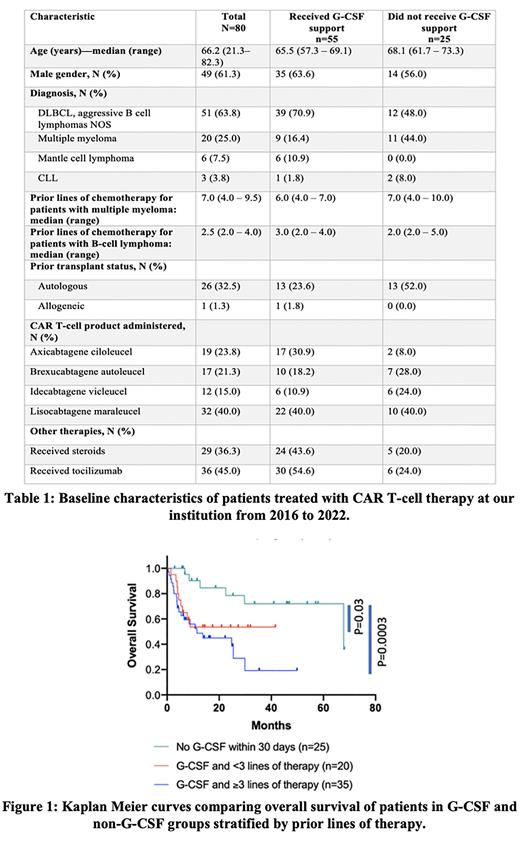
Administration of G-CSF was associated with worse treatment response at day 30, with only 65.5% (36/55) of patients in the G-CSF group attaining complete or partial response, compared to 88% (22/25) of patients in the non-G-CSF group with partial response or better (p=0.04). Patients who received G-CSF had significantly worse PFS (4.43 vs 63.1 months; p=0.01 with HR 2.23 95% CI 1.2 to 4.123 and OS (13.7 vs 67.8 months; p=0.001 with HR 3.62 95% CI 1.88 to 7.02) compared to patients not treated with G-CSF.
On multivariate analyses, differences in survival with G-CSF use persisted despite adjusting for prior lines of therapy, HSCT status, development of CRS and underlying disease for PFS (adjusted HR 3.66; 95% CI 1.67-8.03) and OS (adjusted HR 4.66; 95% CI 1.70-12.76). Although patients with underlying myeloma had worse median OS when compared to patients with lymphoid malignancy (10.8 vs 24.8 months, respectively), both groups had significantly worse OS when compared to patients in the non-G-CSF group (67.8 months, p=0.001 and p=0.002 respectively). Among patients who received G-CSF, three or more prior lines of therapy was associated with worse OS (11.5 months), but patients who received <3 prior lines of therapy also had a significantly worse OS compared to the non-G-CSF group (p=0.03; HR 3.3 with 95% CI 1.1 to 9.8).
Conclusion: In this single institution retrospective study, administration of G-CSF within 30 days of CAR T-cell infusion is associated with worse treatment response, PFS and OS irrespective of underlying disease, prior lines of therapy or HSCT status.
Disclosures
Rosenblatt:Celgene: Research Funding; Merck: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Research Funding; BMS: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Parexel: Consultancy; Imaging Endpoint: Consultancy; Bioclinica: Consultancy; Attivare Therapeutics: Consultancy; Partner Tx: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Dava Oncology: Other: Education; Karyopharm Therapeutics: Other: DSMB; Wolters Kluwer Health Inc: Other: Spouse COI; Kite: Honoraria. Avigan:Celgene: Research Funding; Pharmacyclics: Research Funding; Kite: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Juno: Membership on an entity's Board of Directors or advisory committees; Partners Tx: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Aviv MedTech Ltd.: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Legend Bio Tech: Membership on an entity's Board of Directors or advisory committees; Chugai: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy; Parexel: Consultancy; Takeda: Consultancy; Kite: Consultancy; Sanofi: Consultancy; Kowa: Consultancy. Alonso:Merck: Membership on an entity's Board of Directors or advisory committees; Cidara Therapeutics: Membership on an entity's Board of Directors or advisory committees; AiCuris: Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding. Arnason:Bristol Myers Squibb: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal